Mette Soendergaard • October 18, 2022
Phage display technology is used to select and identify peptides, monoclonal antibodies, or proteins that bind to a specific target molecule. The technique is commonly used in the pharmaceutical industry to develop new drugs such as peptide ligands and antibodies, and in immunology research to study antibody-antigen interactions.
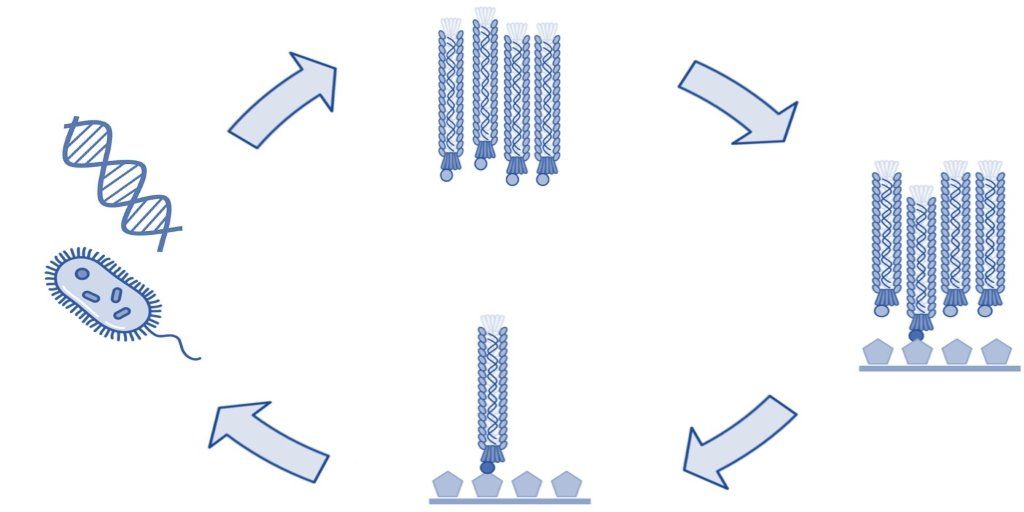
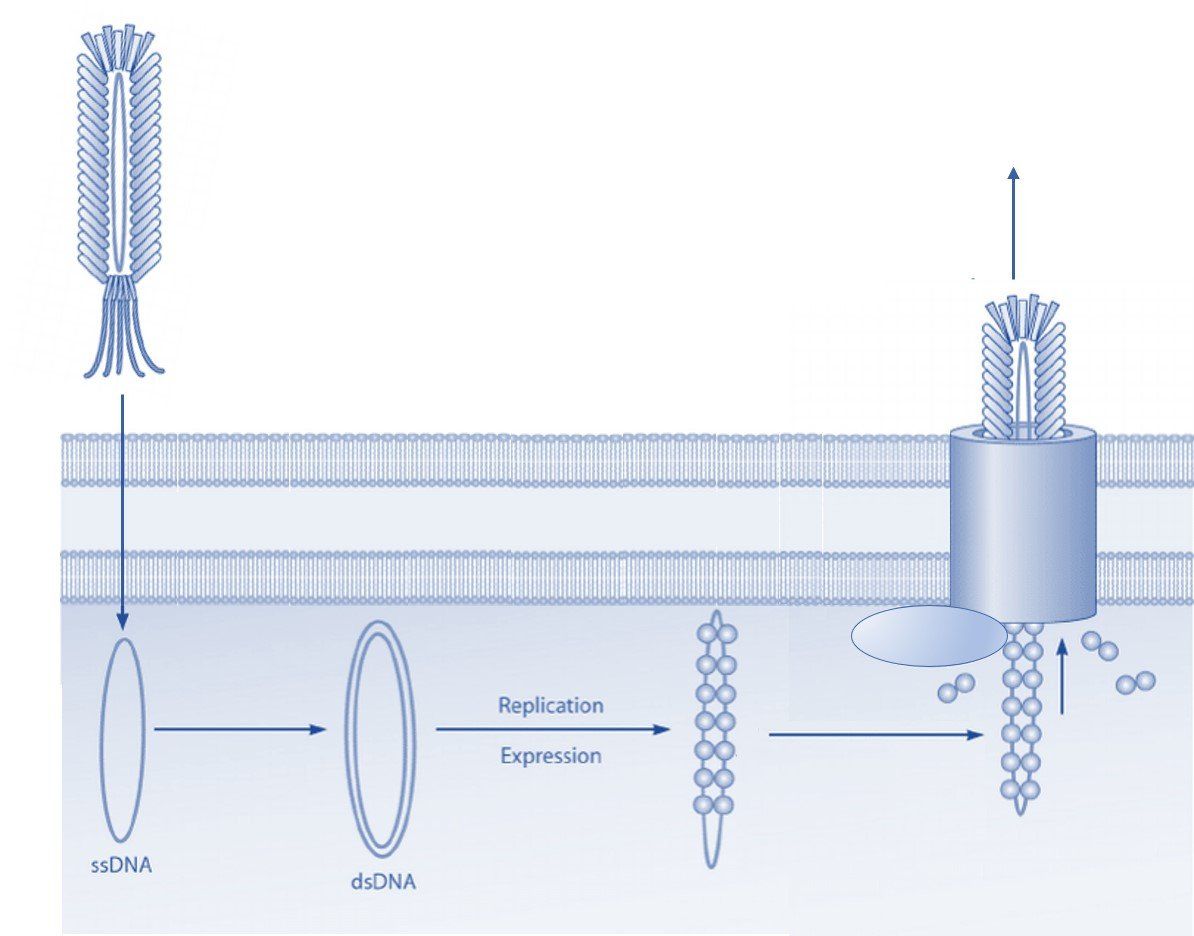
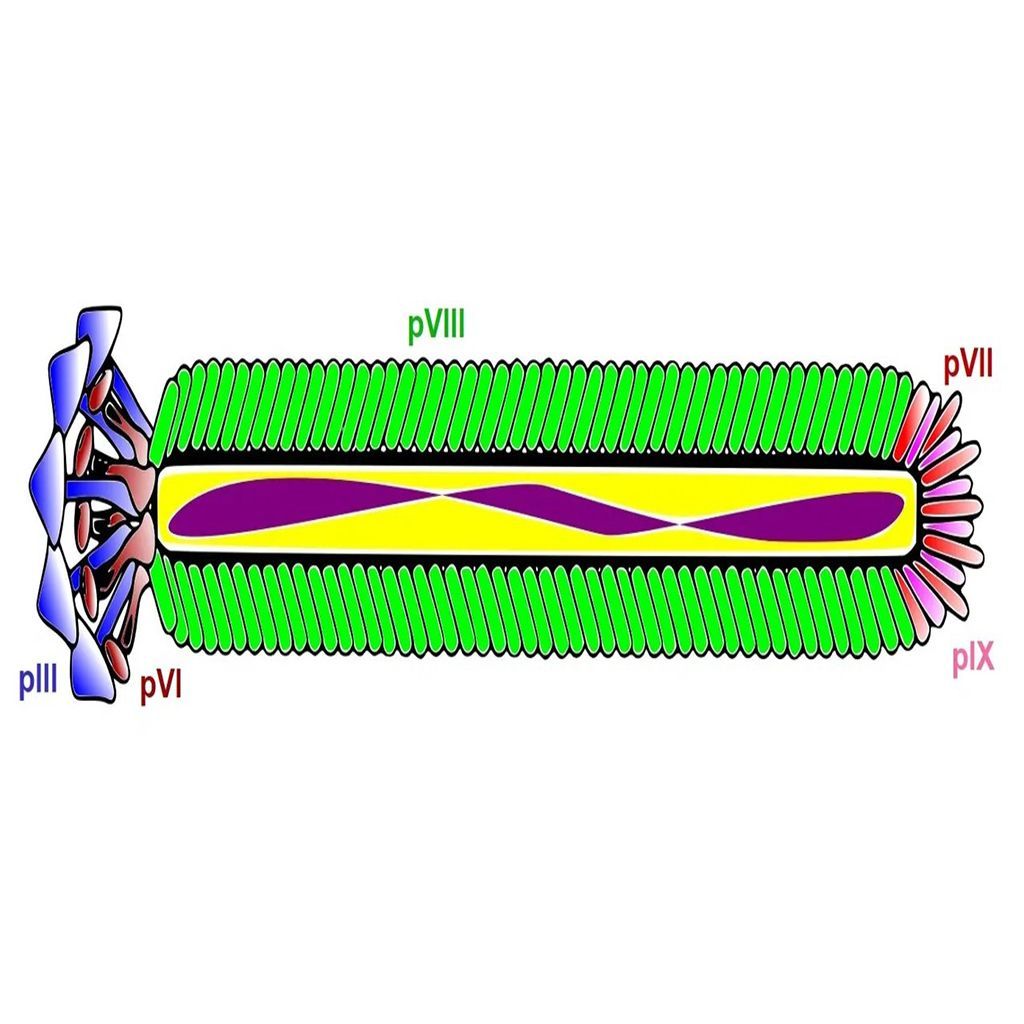
The process of phage display technology involves creating a filamentous fusion phage that displays a random foreign peptide or antibody linked to a phage coat protein. The filamentous phage are then assembled into a phage display library of random peptide sequences or antibodies. These phage display libraries are typically used to select peptide ligands, monoclonal antibodies, or antibody fragments with specific characteristics and binding properties, or used in protein engineering. Here we give an overview of the most common uses of peptide and antibody phage display libraries.
Phage Display Peptide Libraries
Peptide phage display technology is a powerful technique that can be used for a wide variety of applications in research and drug discovery. Peptides can be used directly as drugs to elicit therapeutic effects or to carry cargo to target biomarkers of disease. The small size of peptides offers several advantages as these molecules can extravasate into tissues and can be readily cleared from the body. This is a particular advantage for peptides used in the radiotherapy of cancer where the payload must be delivered directly to the target cells and be rapidly cleared to avoid off-target tissue damage.
Linear Peptides
Both linear and cyclic peptide sequences can be selected using phage display technology and each has its distinct advantages and disadvantages. Linear phage display peptide libraries have been available for decades and are typically cheaper compared to their cyclic counterparts. Additionally, the synthesis of soluble linear peptides is most often easier and more cost-effective, making them a more convenient option for researchers. However, they can have limited bioavailability due to their rapid degradation in the body, which can limit their efficacy as a drug unless they are substantially modified.
Cyclic Peptides
Several novel phage display libraries of cyclic peptides have been developed over the last decade. Such phage-displayed peptide libraries are typically created by inducing disulfide bond formation between cysteine residues, or by using a scaffolding system. Cyclic peptides offer better stability due to their non-natural structure. They also typically offer higher target binding affinity and specificity as a result of their rigid structure. This makes them ideal for applications where high potency or selectivity is required, such as cancer treatments or neurological disorders like Alzheimer's disease. However, the complex synthesis process involved in creating cyclic peptides can be costly and time-consuming, which may make them less desirable for some researchers.
Phage Display Antibody Libraries
Antibody phage display libraries are used in drug discovery and in many research applications. These libraries are often used to screen for antibodies with high binding affinities and specificities for a wide range of targets, including proteins, peptides, small molecules, and whole cells.
Full-Length Monoclonal Antibodies
Full-length monoclonal antibodies have become one of the most important classes in biopharmaceuticals. In fact, therapeutic antibodies continue to dominate markets with six out of ten top-selling drugs belonging to this class. The development of antibody phage display libraries as an alternative technique to the traditional hybridoma technology has accelerated the discovery of monoclonal antibodies. Fully human antibodies derived from large Ig gene repertoires can be assembled in phage display libraries and used to select full-length antibodies with desirable characteristics. The use of a phage display antibody library offers advantages over murine-derived monoclonal antibodies produced by the traditional hybridoma technology that often exhibit limited therapeutic efficiency due to accelerated clearance and allergic reactions.
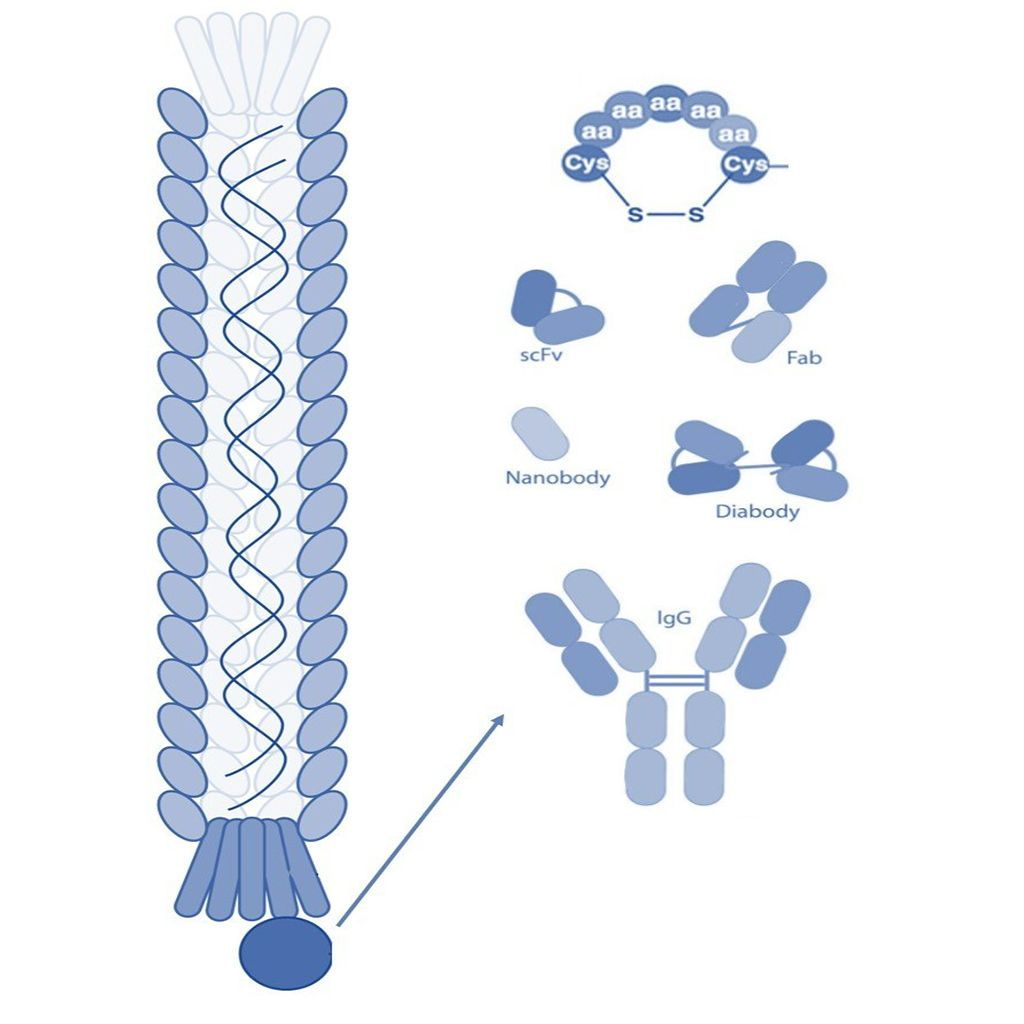
Antibody Fragments
The development of smaller recombinant antibody fragments, such as variable domain (Fv), single-chain variable domain (scFv), diabodies (bivalent scFvs), fragment antigen binding (Fab), and heavy-domain camelid antibody fragments (nanobodies) have helped further advance antibody phage display. These antibody fragments are more amenable to expression than full-length antibodies that require the assembly of four polypeptide chains and extensive disulfide bond formation.
Phage display libraries of human rearranged V-gene repertoires can be constructed from naïve or immunized donors. An advantage of the latter is that they are made from patients who have been immunized with an antigen of interest or who carry a disease, such as a specific cancer type. Thus, a phage display library of this type typically contains a biased antibody repertoire toward a specific target molecule, which can lead to high-affinity antibodies compared to monoclonal antibodies derived from naïve phage display libraries.
On the contrary, a naïve phage display library that is combined from a set of healthy donors contain a more diverse repertoire that can be used to select antibody fragments against an unlimited range of target molecules. Antibody libraries can also be designed synthetically by randomization of the complementarity-determining regions (CDR). For this, random oligonucleotides are synthesized and inserted into the CDRs without disrupting the folding of the V regions thus generating a diverse and completely "naïve" antibody library.
Summary
Phage display technology is a powerful tool that can be used to select peptides and antibodies for use in drug discovery. Peptides and antibodies are vastly different molecules and each offers its own advantages and disadvantages, especially regarding tissue extravasation, immunogenicity, specificity, and binding affinity.
Cell Origins offers premade and custom linear and cyclic phage display peptide libraries as well as antibody phage display libraries with proven high diversity that have resulted in the selection of ligands with optimal binding characteristics. Please contact us to learn more about how we can help with your phage display selections.
Learn More
Alfaleh MA, Alsaab HO, Mahmoud AB, Alkayyal AA, Jones ML, Mahler SM, Hashem AM. Phage Display Derived Monoclonal Antibodies: From Bench to Bedside. Front Immunol. 2020 Aug 28;11:1986. doi: 10.3389/fimmu.2020.01986. PMID: 32983137; PMCID: PMC7485114.
https://www.frontiersin.org/articles/10.3389/fimmu.2020.01986/full
Andrieu J, Re F, Russo L, Nicotra F. Phage-displayed peptides targeting specific tissues and organs. J Drug Target. 2019 Jun-Jul;27(5-6):555-565. doi: 10.1080/1061186X.2018.1531419. Epub 2018 Oct 24. PMID: 30281393.
https://www.tandfonline.com/doi/full/10.1080/1061186X.2018.1531419
Bazan J, Całkosiński I, Gamian A. Phage display--a powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum Vaccin Immunother. 2012 Dec 1;8(12):1817-28. doi: 10.4161/hv.21703. Epub 2012 Aug 21. PMID: 22906939; PMCID: PMC3656071.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3656071/
Kumar R, Parray HA, Shrivastava T, Sinha S, Luthra K. Phage display antibody libraries: A robust approach for generation of recombinant human monoclonal antibodies. Int J Biol Macromol. 2019 Aug 15;135:907-918. doi: 10.1016/j.ijbiomac.2019.06.006. Epub 2019 Jun 3. PMID: 31170490.
https://www.sciencedirect.com/science/article/abs/pii/S0141813019330855?via%3Dihub
Ledsgaard L, Kilstrup M, Karatt-Vellatt A, McCafferty J, Laustsen AH. Basics of Antibody Phage Display Technology. Toxins (Basel). 2018 Jun 9;10(6):236. doi: 10.3390/toxins10060236. PMID: 29890762; PMCID: PMC6024766.
https://www.mdpi.com/2072-6651/10/6/236
Saw PE, Song EW. Phage display screening of therapeutic peptide for cancer targeting and therapy. Protein Cell. 2019 Nov;10(11):787-807. doi: 10.1007/s13238-019-0639-7. Epub 2019 May 28. PMID: 31140150; PMCID: PMC6834755.
https://link.springer.com/article/10.1007/s13238-019-0639-7
Sioud M. Phage Display Libraries: From Binders to Targeted Drug Delivery and Human Therapeutics. Mol Biotechnol. 2019 Apr;61(4):286-303. doi: 10.1007/s12033-019-00156-8. PMID: 30729435.
https://link.springer.com/article/10.1007/s12033-019-00156-8
Wu CH, Liu IJ, Lu RM, Wu HC. Advancement and applications of peptide phage display technology in biomedical science. J Biomed Sci. 2016 Jan 19;23:8. doi: 10.1186/s12929-016-0223-x. PMID: 26786672; PMCID: PMC4717660.
https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-016-0223-x