Mette Soendergaard • January 24, 2023

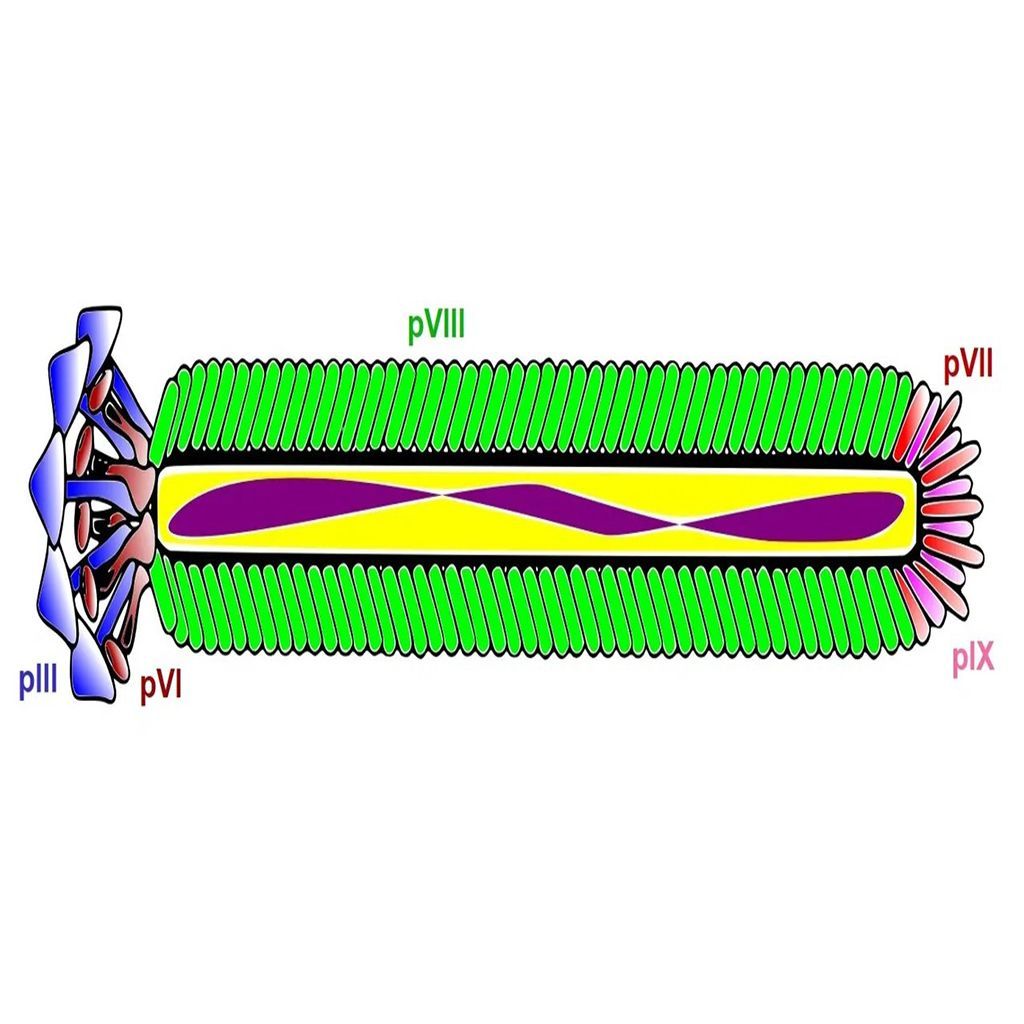
Phage display technology
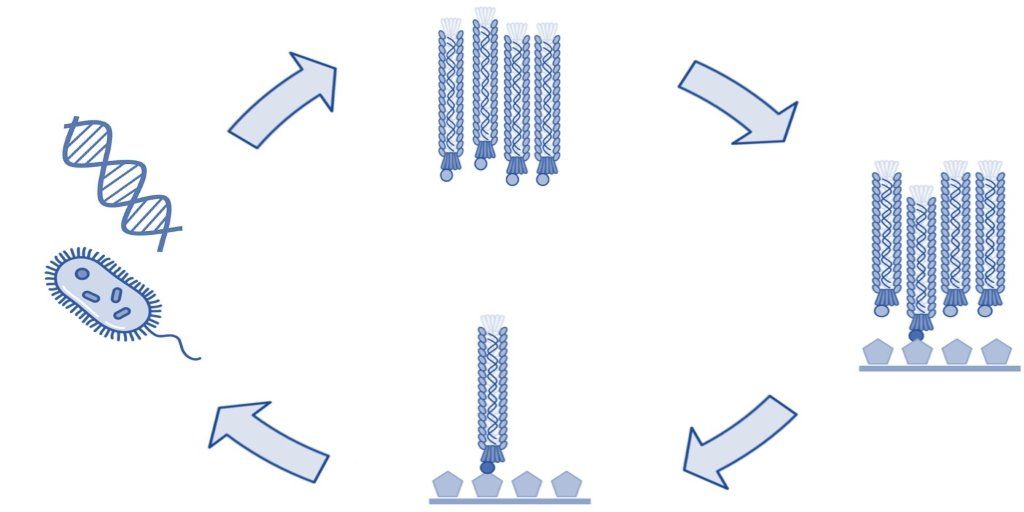
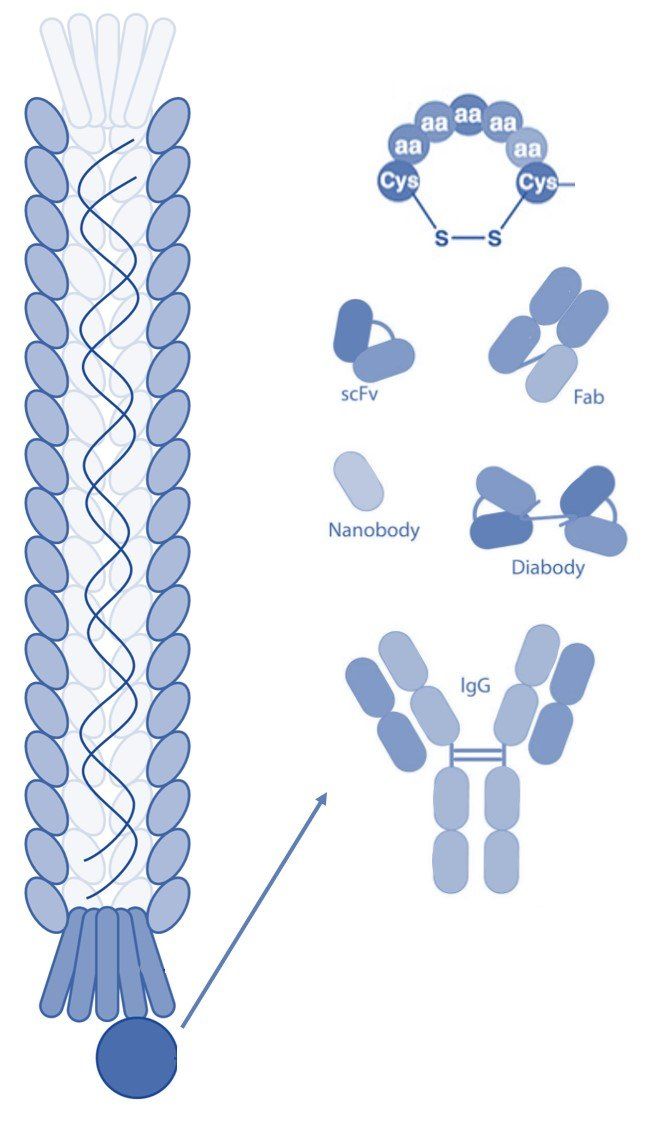
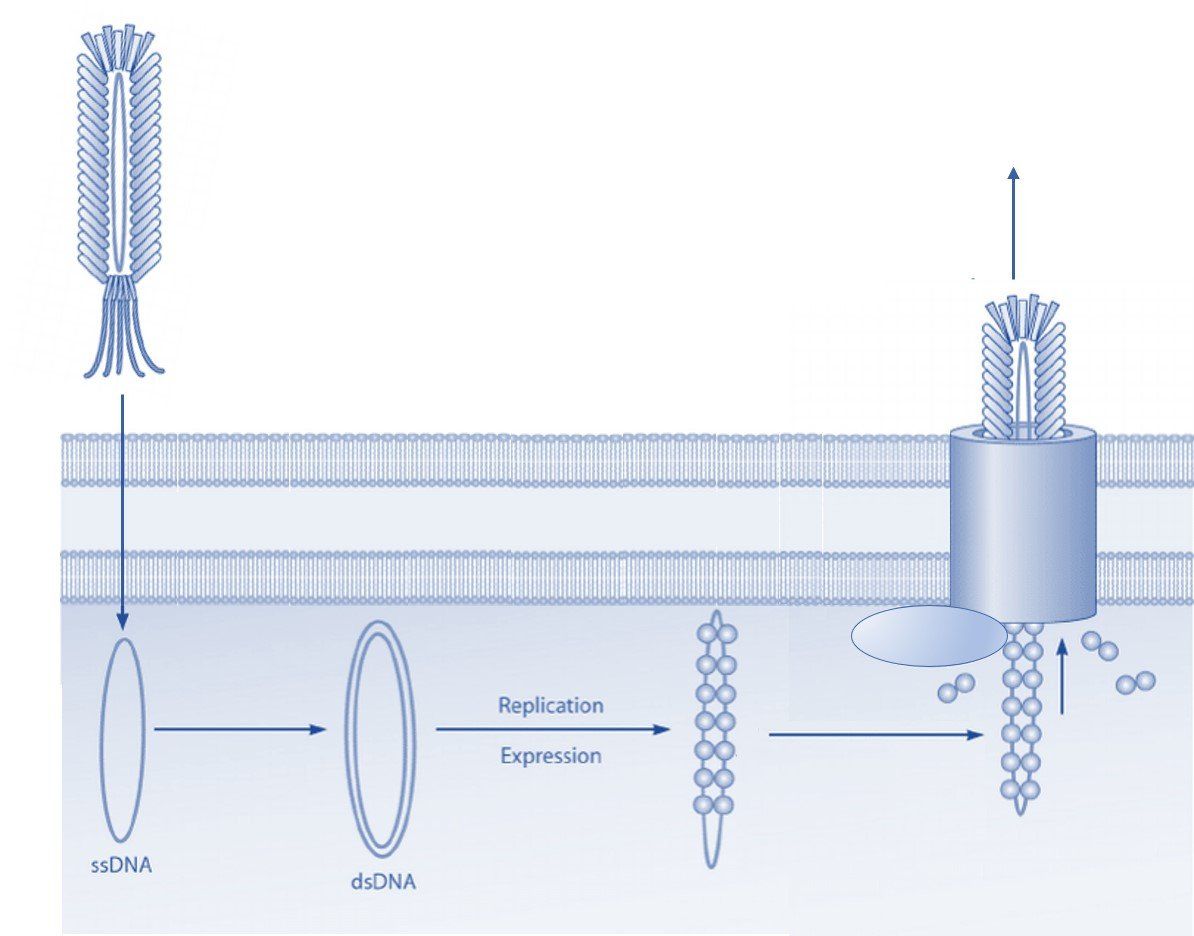
Phage display technology is a powerful tool used in drug discovery to develop peptides and monoclonal antibodies that bind specifically with high affinity to a target molecule. The technique involves genetically engineering bacteriophages to display foreign peptides or antibodies on a viral coat protein to create a filamentous fusion phage. Phage display libraries are collections of such phages that typically contain 10^9-10^10 different phage clones, making them a powerful tool for screening and discovering new targeting molecules. Due to their flexible design and large-scale production capabilities, phage display libraries have become an important tool in the drug discovery process, allowing scientists to rapidly screen for potential new drug targets and candidate compounds. Additionally, phage display technology has been successfully employed in several other areas of scientific research, including immunology and protein engineering. Here we give a brief overview of the key advantages of phage display in drug discovery.
Phage Display is Highly Versatile
Phage display can be used for a variety of applications at different stages in drug development including peptide and antibody discovery, drug candidate screening, and lead optimization. Additionally, phage display libraries can be screened using a variety of different biopanning methodologies depending on the needs of the researcher. Biopanning involves iterative rounds of affinity selection against a target molecule, cell, or tissue to select phages that display peptides or antibodies with certain desired binding and pharmacokinetic characteristics. During biopanning, a phage library is exposed to the target molecule, and unbound or weakly bound phages are removed by washing, after which bound phage particles are collected by elution.
Phage display biopanning is versatile because almost any type of peptide or antibody library can be screened against any type of target. For in vitro biopanning experiments, the phage display library is typically affinity selected against recombinant proteins or other isolated molecules. This type of selection often results in the identification of high-affinity binders. However, many studies have shown that peptides and antibodies selected by in vitro phage display often fail to exhibit optimal binding in live tissues and whole organisms and demonstrate less-than-ideal pharmacokinetic properties. Instead, more researchers are recognizing that phage display selections against cells and tissues (in situ) and in live animals or patients (in vivo) offer advantages regarding the identification of ligands that show high binding affinity, minimal off-target binding, and optimal pharmacokinetics. This is especially useful when interrogating a complex biological process that cannot easily be replicated in vitro, such as differentially expressed target proteins of varying lengths and accessibility, as well as ones that have different binding partners depending on the tissue, as is often observed in normal vs. tumor tissues. At Cell Origins, we are pioneers in utilizing multi-tier phage display techniques to develop peptides and monoclonal antibodies that show desirable binding kinetics and pharmacokinetics. Our platform ensures that lead compounds maintain their high binding affinity and specificity while having optimal pharmacokinetics and minimal off-target binding.
Phage Display is High Throughput
The phage display platform is highly efficient and can screen billions of different peptides or antibodies in a single day. This high throughput capacity makes phage display an attractive technology in drug discovery. Practically, this means that phage display selections can be used to screen large libraries in a matter of a few days and that attractive drug candidates can be identified in as little as a few weeks after next-generation sequencing and bioinformatic analysis have been completed. Furthermore, using filamentous phage to display desirable ligands offer the ability to screen and analyze candidates in a high throughput and cost-effective manner that is not possible with individual soluble peptides and antibodies. As such, recombinant DNA technology, rapid propagation in E. coli, and the development of easy tagging methods allow kinetic analyses to be carried out post-selection in a much more rapid manner. While the synthesis of peptide sequences and the production of monoclonal antibodies typically take weeks to complete, the production of phage for kinetic analyses can be carried out in a few days.
Phage Display is Cost-Effective
Phage display screening is among the least expensive investigation techniques available for drug discovery. Compared with other technologies, phage display selections typically require fewer resources, reagents, and consumables while generating targeting ligands with high-affinity and desirable binding properties in a short amount of time. Additionally, phage libraries are known to be highly robust and can be stored for extended periods of time without a significant loss in infectivity and usability.
The cost-effectiveness and versatility of phage display technology allow the researcher to simultaneously interrogate several types of antibody and peptide libraries against potential biomarkers under different conditions. Such a multiplexing approach lowers the overall cost of drug discovery and further provides an opportunity to add additional levels of competition and complexity to the phage display selection to more rapidly generate ligands with optimal properties.
Summary
Phage display provides a versatile and powerful tool to rapidly identify high-affinity ligands in a cost-effective manner. At Cell Origins, we are experts in phage display with extensive experience in generating peptides and antibodies that bind difficult and complex targets both in vitro and in vivo. Our multi-tier phage display selection platform is proven to result in ligands that exhibit high affinity, and excellent pharmacokinetics while showing minimal off-target binding. Please contact us to learn more about how our unique phage display platform can accelerate your drug discovery.
Learn More
Biopanning
https://en.m.wikipedia.org/wiki/Biopanning
Phage Display Derived Monoclonal Antibodies: From Bench to Bedside.
https://www.frontiersin.org/articles/10.3389/fimmu.2020.01986/full
Phage-displayed peptides targeting specific tissues and organs.
https://www.tandfonline.com/doi/full/10.1080/1061186X.2018.1531419
Phage display--a powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3656071/
Phage display antibody libraries: A robust approach for generation of recombinant human monoclonal antibodies.
https://www.sciencedirect.com/science/article/abs/pii/S0141813019330855?via%3Dihub
Basics of Antibody Phage Display Technology.
https://www.mdpi.com/2072-6651/10/6/236
Phage display screening of therapeutic peptide for cancer targeting and therapy.
https://link.springer.com/article/10.1007/s13238-019-0639-7
Phage Display Libraries: From Binders to Targeted Drug Delivery and Human Therapeutics.
https://link.springer.com/article/10.1007/s12033-019-00156-8
Advancement and applications of peptide phage display technology in biomedical science.
https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-016-0223-x